Search OneLab Network Past Events

CLIA Proficiency Testing (PT) Final Rule, CMS-3355-F
This presentation will provide information on the CLIA Proficiency Testing (PT) Final Rule which was published in the Federal Register Notice on July 11, 2022.
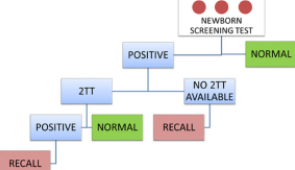
Oregon State Public Health Laboratory Shares Lessons Learned from Its Newborn Screening Program
The history of newborn screening (NBS) demonstrates the strength and integrity of its mission: to identify children with congenital conditions who need treatment to prevent adverse outcomes.

Biosafety Practices and Reporting Occupational Exposures for Select Agents and Toxins (Part 2)
This basic-level webinar is designed to help clinical and veterinary laboratory professionals understand how to report select agent and toxin identifications and recognize and report exposure incidents.

Recognizing, Identifying, and Reporting the Identification of Select Agents and Toxins
This beginner-level webinar is for clinical and veterinary laboratory professionals to understand how to report select agent and toxin identifications and recognize and report exposure incidents.
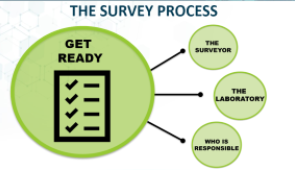
The Survey Process: What You Need to Know for Your CLIA Survey
Preparing for laboratory survey? Laboratory staff are invited to join this webinar, where Marranda Scott, a Clinical Laboratory Scientist with the CDC Division of Laboratory Systems, will review processes and requirements for a CLIA laboratory survey.

Pipeline and Hazardous Materials Safety Administration (PHMSA) Training Program Requirements: Shipping Hazmat such as Cat A
The U.S. Department of Transportation provides helpful information about creating and implementing a laboratory hazmat plan.

Navigating Laboratory Education and Training Needs: An Open Forum
Start your year by collaborating and sharing your training and education needs through CDC’s OneLab Network. Do you have laboratory guidance needs? Are there training materials you are interested in developing for your laboratory staff?

Laboratory Training: Virtual Reality (VR)
Please join us for our next OneLab Network meeting on Friday, December 16 at 12 pm EST.
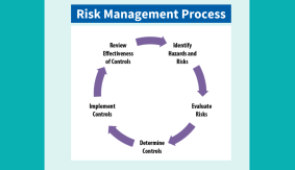
I’m a Frontline Facility – How Can I Safely Test Clinical Specimens From a Suspected Ebola Patient
Join us for our next OneLab™ Network Event in which will give an overview of the current Sudan Ebolavirus outbreak and discuss testing options for frontline facilities, identifying potential hazards in laboratory processes, and risk mitigation strategies for safe laboratory test

When Receiving Samples from Patients Suspected to be Infected with Ebola: What’s the Plan?
Learn how clinical and public health laboratories collaborate with healthcare teams to provide the testing required to diagnose and treat hospital patients under investigation (PUI) for Ebola virus disease.
